Join Raybow at DCAT Week in New York City!
Privileged Chiral Spiro Ligands and Applications to Commercially Marketed APIs
Raybow PharmaScience (Raybow) is pleased to highlight the use of privileged chiral spiro ligands for asymmetric synthesis with successful application to a number of commercially marketed APIs. The key is more than 200 privileged chiral ligands with high enantioselectivity, high turnover number, high stability, ease of handling and readily tunable that Raybow has licensed from a number of leading academic researchers, specifically Professors Qilin Zhou (Nankai University) and Wenjun Tang (Shanghai Institute of Organic Chemistry).
It is important to note that these privileged chiral spiro ligands are free and clear of any Intellectual Property issues and their use will not require any royalty payments from Raybow’s clients.
These privileged chiral spiro ligands can be applied to a number of chemical transformations including asymmetric hydrogenation, asymmetric carbonyl reductions, cross-coupling (C-C, C-N, C-B), hydroboration/diboration and nucleophilic addition reactions.
Raybow has applied these privileged chiral ligands to a number of client projects, ranging from Process R&D to metric tonne manufacturing campaigns. Examples include Crizotinib (asymmetric reduction of a carbonyl intermediate; 100’s of Kg per annum), Ensartinib (asymmetric reduction of a carbonyl intermediate; MT per annum), Rivastigmine (asymmetric reduction of a carbonyl intermediate, 100’s of Kg per annum) and Aleglitazar (asymmetric hydrogenation; clinical supplies to Phase III). For all these examples more concise and/or higher yielding manufacturing processes with lower costs were developed.
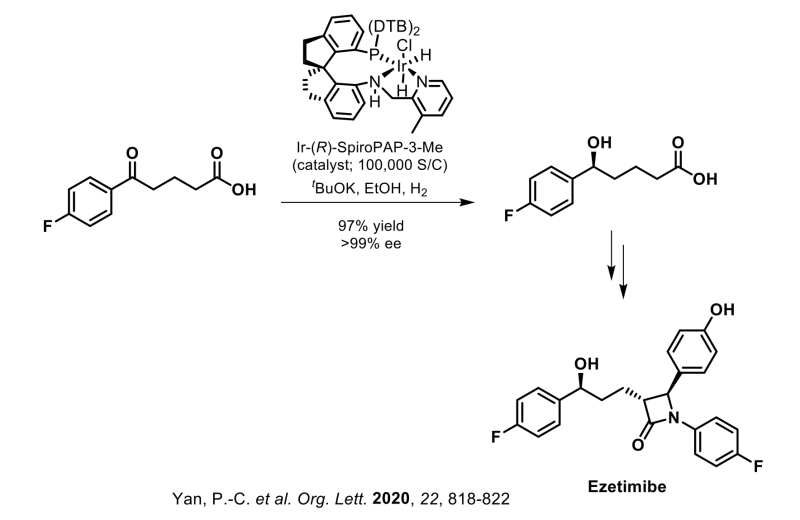
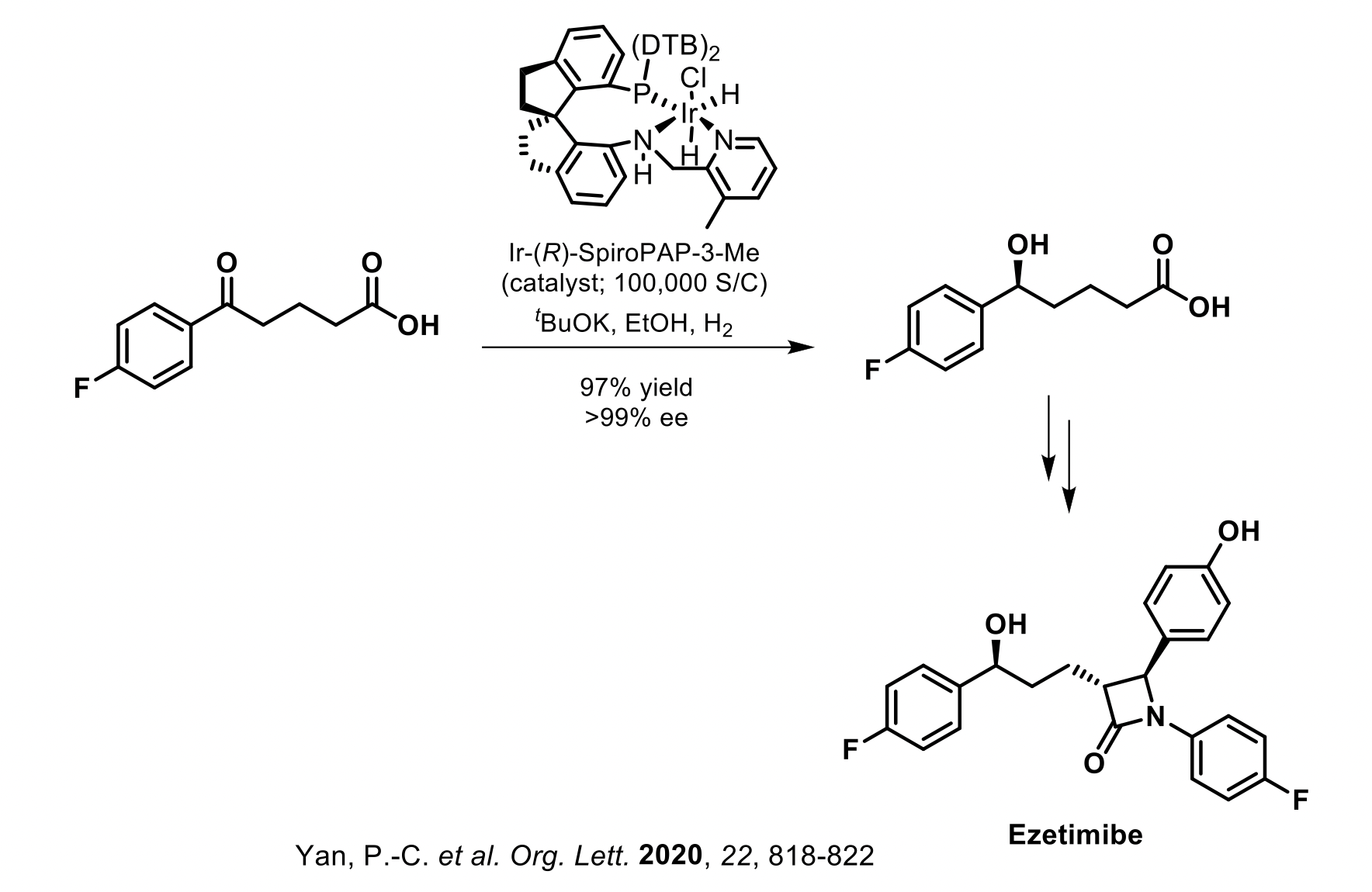
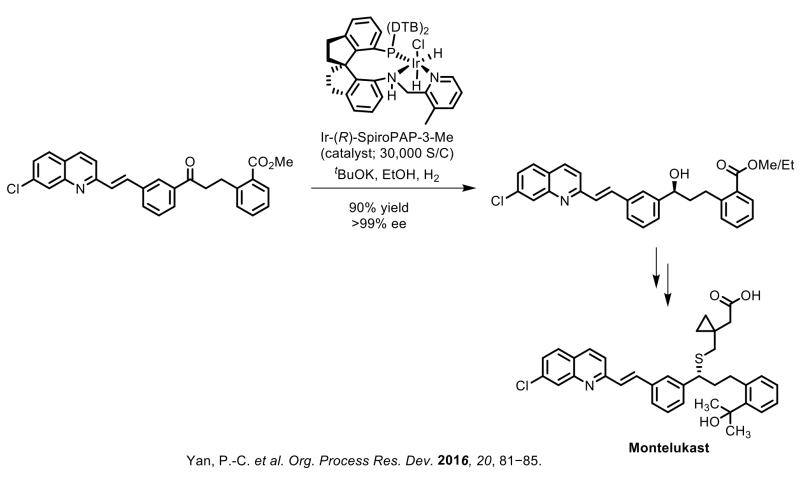
Raybow has further demonstrated the viability of these privileged chiral spiro ligands by their application to a number of other commercially marketed APIs at gram to kilo-gram scale. These APIs include Montelukast (asymmetric reduction of a ketoester intermediate), Ezetimibe (asymmetric reduction of a ketoacid intermediate), Clopidogrel (asymmetric reduction of a ketoacid intermediate), Dapoxetine (asymmetric reduction of a beta-ketoester intermediate), Empagliflozin (asymmetric reduction of a lactone intermediate) and Silodosin (asymmetric hydrogenation of an olefin intermediate).
Raybow will be happy to discuss the potential applicability of these privileged chiral spiro ligands for your chemistry needs.
Meet us at ChemOutsourcing (September 8 and 9, Parsippany, NJ, USA), BioEurope in Leipzig (Oct 24 – 26) or CPhI Europe (November 1-3, 2022, Frankfurt, Germany).
Other Raybow News & Papers
Raybow 2024 Show Schedule
Join us October 24th thru the 26th in Barcelona.
Join us July 4th and 5th in Basel
Join us in Savannah for this annual showcase










