Jiuzhou Pharma Appoints Industry Leader Mike Pennington as Global Head of TIDES
Green Chemistry in the development and manufacturing of pharmaceuticals
First of all, Green Chemistry is “the development of chemical products and processes to reduce or eliminate the use and generation of hazardous substances” and “is intended to apply to the development and full lifecycle of a chemical product” i.e. development, manufacturing, application and disposal. This definition is given by the EPA (the US Environmental Protection Agency1) and can be applied to small molecule pharmaceuticals as well. There are 12 principles of green chemistry defined by the EPA, applied they prevent dangerous raw materials, intermediates and reagents from getting into the environment.
I. Prevent waste: Design chemical syntheses to prevent waste. Leave no waste to treat or clean up.
II. Maximize atom economy: Design syntheses so that the final product contains the maximum proportion of the starting materials. Waste few or no atoms.
III. Design less hazardous chemical syntheses: Design syntheses to use and generate substances with little or no toxicity to either humans or the environment.
IV. Design safer chemicals and products: Design chemical products that are fully effective yet have little or no toxicity.
V. Use safer solvents and reaction conditions: Avoid using solvents, separation agents, or other auxiliary chemicals. If you must use these chemicals, use safer ones.
VI. Increase energy efficiency: Run chemical reactions at room temperature and pressure whenever possible.
VII. Use renewable feedstocks: Use starting materials (also known as feedstocks) that are renewable rather than depletable. The source of renewable feedstocks is often agricultural products or the wastes of other processes; the source of depletable feedstocks is often fossil fuels (petroleum, natural gas, or coal) or mining operations.
VIII. Avoid chemical derivatives: Avoid using blocking or protecting groups or any temporary modifications if possible. Derivatives use additional reagents and generate waste.
IX. Use catalysts, not stoichiometric reagents: Minimize waste by using catalytic reactions. Catalysts are effective in small amounts and can carry out a single reaction many times. They are preferable to stoichiometric reagents, which are used in excess and carry out a reaction only once.
X. Design chemicals and products to degrade after use: Design chemical products to break down to innocuous substances after use so that they do not accumulate in the environment.
XI. Analyze in real time to prevent pollution: Include in-process, real-time monitoring and control during syntheses to minimize or eliminate the formation of byproducts.
XII. Minimize the potential for accidents: Design chemicals and their physical forms (solid, liquid, or gas) to minimize the potential for chemical accidents including explosions, fires, and releases to the environment.
Today, in the early stages of drug development, too little emphasis is placed on these principles of Green Chemistry. There are many reasons for this, most of all, drug development is about speed. Nevertheless, process strategies based on green chemistry are inherently simple and therefore more adaptable over time, making green chemistry economically viable.
As a CDMO, we at Raybow Pharmaceutical attach great importance to developing new technologies for environmentally friendly production. One of these new technologies is continuous flow which allows us to reduce the use of solvents and to control dangerous reactions in a more effective way.
The following case demonstrates the influence of the principles of Green Chemistry. In an early phase of development, we investigated a Curtius rearrangement (i.e. thermal decomposition of an azide to a primary amine) with a safety rating that showed high exothermic, difficult to control reaction conditions, especially on production scale.
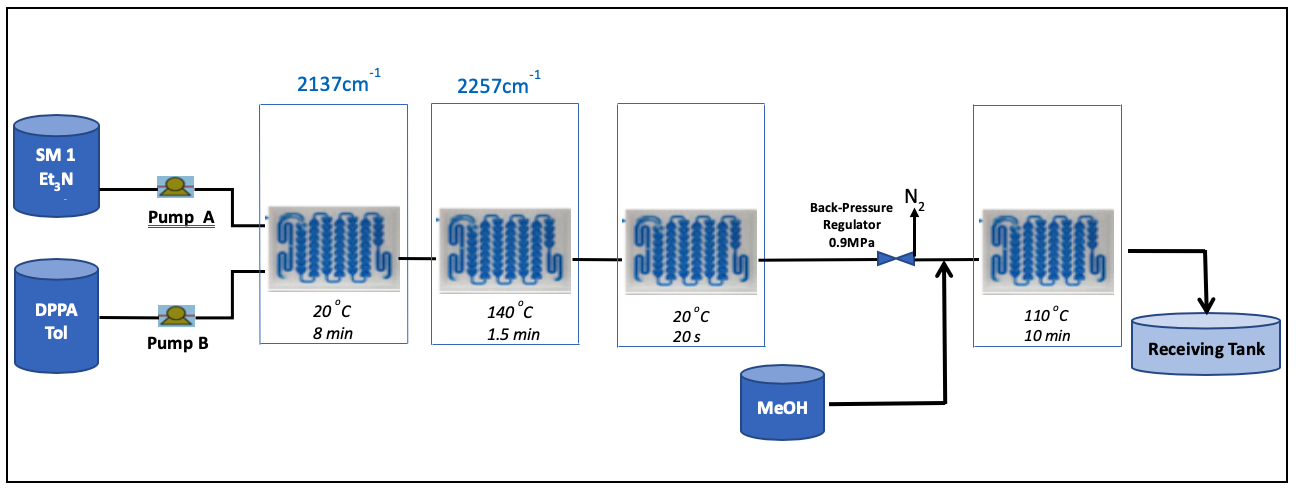
As an alternative reaction path using a Hofmann reaction would have led to rising costs, and it would also have created new impurities that would not have been tolerable in this development phase. For this reason, we developed a continuous flow reaction for the specific Curtius reaction, first on laboratory scale using a Corning G1.
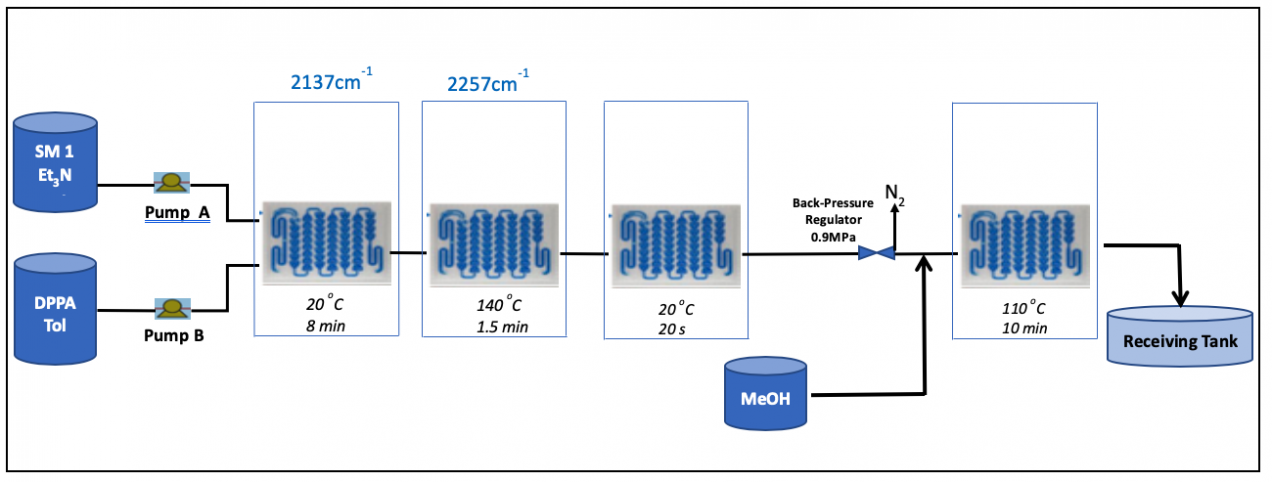
Then our own system was installed for the small production scale of 800 kg of the final product.
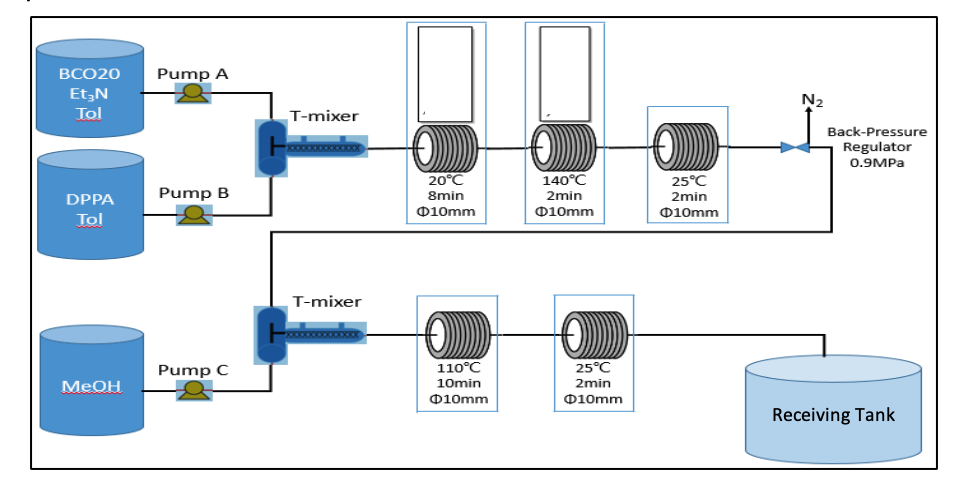
As a result, we achieved a more efficient process compared to batch production. Among the numerous benefits are
- Higher quality of the final product, less impurities
- Solvent consumption could be reduced by 60 %, which not only reduces expenses but also contributes to protecting the environment
- Significant increase in safety. This is very important as the original goal was a higher process safety where less hazardous reaction conditions - in this case the amount of material in the exothermic reaction - are controllable in a better way
- Overall production cost could be reduced by 30 %. This is mainly due to less solvent, time and manpower to perform the synthesis
The example illustrates that Green Chemistry – in this case applying principles I, V, XI and XII – helps to reduce costs, protect the environment and therefore adds value to our customers supply chain. Following the principles of Green Chemistry is a competitive edge for Raybow Pharmaceutical.
Dr. Dirk Hütten, Senior Director Business Development, Raybow Pharmaceutical
Other Raybow News & Papers
Lauer tapped as new Raybow USA president
Join us October 24th thru the 26th in Barcelona.
Join us July 4th and 5th in Basel










