Nucleotide Services
Team

Nucleic Acid Chemistry Capabilities
Process Research, Development, and Optimization
- Process development of discovery routes/early development routes into robust manufacturing processes suitable for intended purpose
- Route scouting/selection for new scalable processes, including generation of innovative solutions to complex Oligonucleotide synthetic problems
- Process characterization (Quality by Design)
- Technology transfer to manufacturing production
- Custom Synthesis
- Drug discovery & Preclinical development support
- CMC CTD Support
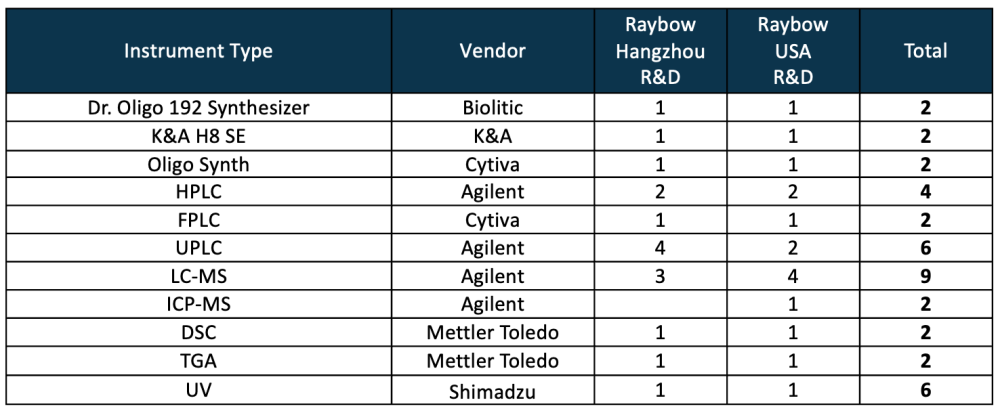
Key Oligonucleotide Synthesis Instruments
Oligonucleotide Synthesis Capabilities
Process Research, Development, and Optimization
- Small-scale oligonucleotide synthesis (1-10 mg) capability (R&D and pre-clinical study) 200-400 oligos/day (depending on the type of oligonucleotides).
- Mid-scale oligonucleotide synthesis (10-100g) 2-4 oligos/month
- Critical process parameter evaluation/justification
- Technology transfer to manufacturing production
- Custom Synthesis
- Drug discovery & Preclinical development support
- Impurity markers
- Comparators
- CMC CTD Support
- Registration Starting Material Strategy
- Impurity management – fate and linkage
- Potential Genotoxic Impurity Management
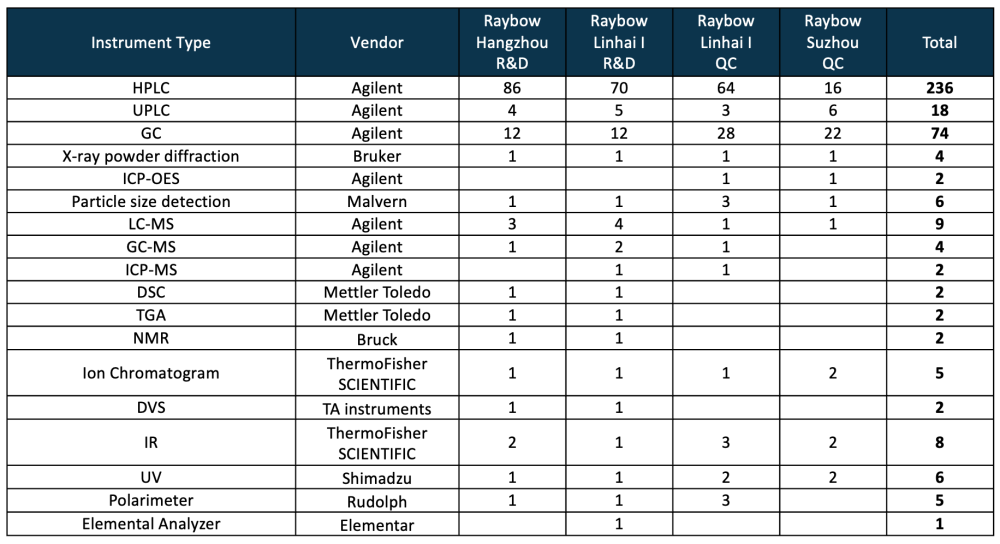
Key Analytical Instruments
Nucleotide Analytical Chemistry Capabilities
Analytical Development Chemistry
- Oligonucleotide synthesis Projects
- Assay Method Development and Validation
- Impurity Characterizations
- In-Process Controls
- Purification
- Structural Characterizations
- Physical & Chemical Characterizations and General Testing